How to determine the oxidation state. Algorithm for determining the oxidation state and valence of an element in a compound. The highest oxidation state of which element
Definition
Electronegativity (EO) $\chi$(chi) is a quantity characterizing the ability of an atom of an element to attract electrons to itself when forming a chemical bond with other atoms.
The modern concept of electronegativity of atoms was introduced by the American scientist Linus Pauling in 1932. The theoretical definition of electronegativity was developed later. American physicist Robert Mulliken proposed calculating electronegativity as half the sum of the ionization potential and electron affinity:
$\chi_(\textrm(M)) = \dfrac (I + A_e)(2),$
where $I$ is the ionization potential, $A_e$ is the electron affinity energy.
In addition to the Mulliken scale described above, there are more than 20 different other electronegativity scales (the calculation of the values of which is based on different properties of substances), including the L. Pauling scale (based on the binding energy during the formation of a complex substance from simple ones), the Allred scale Rokhov (based on the electrostatic force acting on an external electron), etc.
Currently, there are many ways to quantify the electronegativity of an atom. Electronegativity values of elements calculated different ways, as a rule, do not coincide even with the introduction of correction factors. However, the general trends in changes in $\chi$ according to the Periodic System remain. This can be illustrated by comparing the two most widely used scales - Pauling and Allred-Rochow ( bold EO values on the Pauling scale are highlighted in font, italics- according to the Allred-Rochov scale; $s$-elements are highlighted in pink, $p$-elements are in yellow, $d$-elements are in green, $f$-elements are in blue):


Strictly speaking, an element cannot be assigned constant electronegativity. The electronegativity of an atom depends on many factors, in particular, on the valence state of the atom, the formal oxidation state, the type of compound, coordination number, the nature of the ligands that make up the environment of the atom in the molecular system, and some others.
Electronegativity is related to the redox activity of an element. Accordingly, the greater the electronegativity of an element, the stronger its oxidizing properties.
The closer the electron shell of a given atom is to the electron shell of an inert gas, the higher its electronegativity. In other words, in periods As the outer energy level is filled with electrons (that is, from left to right), electronegativity increases, as the group number and the number of electrons in the outer energy level increase.
The further the valence electrons are from the nucleus, the weaker they are held and the lower the atom's ability to attract additional electrons. Thus, in groups electronegativity increases with decreasing atomic radius, that is, from bottom to top. The element with the highest electronegativity is fluorine, and the element with the least is cesium. Typical nonmetals thus have high electronegativity values, while typical metals have low electronegativity values.

VALENCE OF CHEMICAL ELEMENTS
Valence characterizes the ability of atoms of a given chemical element to form chemical bonds.
Valence determines the number of chemical bonds by which an atom is connected to other atoms in a molecule.
Previously, valency was defined as the number of atoms of a monovalent element with which one atom of a given element combines. Thus, hydrogen is considered a monovalent element. In the $HBr$ molecule, the bromine atom is combined with one hydrogen atom, and the sulfur atom in the $H_2S$ molecule is combined with two hydrogen atoms. Consequently, bromine in $HBr$ is monovalent, and sulfur in $H_2S$ is divalent. Valency values for different elements can vary from one to eight. Thus, in perchloric acid $HClO_4$ the element hydrogen is monovalent, oxygen is divalent, chlorine is heptavalent. In the xenon oxide $XeO_4$ molecule, the xenon valence reaches a value of eight. All this is clearly demonstrated by the following structural formulas, which show the order of bonding of atoms in a molecule with each other in accordance with their valencies (with each valence unit corresponding to one valence line):

Definition
Currently under valency understand the number of electron pairs with which a given atom is connected to other atoms.
Valence(or covalency) determined by the number of covalent bonds formed by a given atom in a compound. In this case, both covalent bonds formed by the exchange mechanism and covalent bonds formed by the donor-acceptor mechanism are taken into account.
Valence has no sign!
Since there are two mechanisms for the formation of a covalent bond (the electron pairing mechanism and the donor-acceptor mechanism), the valence capabilities of atoms depend on:
- the number of unpaired electrons in a given atom;
- from the presence of vacant atomic orbitals in the outer level;
- on the number of lone electron pairs.
The valence of elements of the first period cannot exceed I, the valence of elements of the second period cannot exceed IV. Starting from the third period, the valence of elements can increase to VIII (for example, $XeO_4$) in accordance with the number of the group in which the element is located.
Let us consider, for example, the valence possibilities of atoms of a number of elements.
VALENCE POSSIBILITIES OF HYDROGEN
The hydrogen atom has a single valence electron, which is reflected by the electronic formula $1s^1$ or graphical formula:

Due to this unpaired electron, a hydrogen atom can form only one covalent bond with any other atom through the mechanism of pairing (or sharing) of electrons. The hydrogen atom has no other valence possibilities. Therefore, hydrogen exhibits a single valence of I.
VALENCE POSSIBILITIES OF PHOSPHORUS
The element phosphorus is in the third period, in the main subgroup of the fifth group. The electronic configuration of its valence electrons is $3s^23p^3$ or

Being an analogue of nitrogen, phosphorus can also exhibit valences I, II, III and IV. But since vacant $3d$ orbitals are available for elements of the third period, the phosphorus atom can go into an excited state by transferring one of the $s$ electrons to the $d$ sublevel:

Thus, a phosphorus atom can form five covalent bonds by an exchange mechanism. Phosphorus exhibits the maximum valency V in molecules $PF_5$, $H_3PO_4$, $POCl_3$, etc.:

OXIDATION STATE
Definition
Oxidation state is the conditional charge of an atom in a compound, assuming that all bonds in this compound are ionic (that is, all bonding electron pairs are completely shifted towards the atom of the more electronegative element).
In other words, the oxidation number is a number that indicates how many electrons an atom gives up (+ charge) or accepts (– charge) when forming a chemical bond with another atom.
Unlike valency, the oxidation number has a sign - it can be negative, zero or positive.
To calculate the oxidation states of atoms in a compound, there are a number of simple rules:
- The oxidation state of an element in a simple substance is assumed to be zero. If a substance is in an atomic state, then the oxidation state of its atoms is also zero.
- A number of elements exhibit a constant state of oxidation in compounds. Among them are fluorine (−1), alkali metals (+1), alkaline earth metals, beryllium, magnesium and zinc (+2), aluminum (+3).
- Oxygen, as a rule, exhibits an oxidation state of −2, with the exception of peroxides $H_2O_2$ (−1), superoxides $MO_2$ ($-\frac(1)(2)$), ozonides $M^IO_3,\ M^(II )(O_3)_2$ ($-\frac(1)(3)$) and oxygen fluoride $OF_2$ (+2).
- Hydrogen in combination with metals (in hydrides) exhibits an oxidation state of −1, and in compounds with non-metals, as a rule, +1 (except $SiH_4,\ B_2H_6$).
- The algebraic sum of the oxidation states of all atoms in a molecule must be equal to zero, and in a complex ion - the charge of this ion.
Highest positive oxidation state equal, as a rule, to the group number of the element in the periodic table.
Thus, sulfur (an element of group VIA) exhibits the highest oxidation state of +6, nitrogen (an element of group V) - the highest oxidation state of +5, manganese - a transition element of group VIIB - the highest oxidation state of +7. This rule does not apply to elements of the side subgroup of the first group, the oxidation states of which usually exceed +1, as well as to elements of the side subgroup of group VIII. The elements oxygen and fluorine also do not show their highest oxidation states equal to the group number.
Lowest negative oxidation state for non-metal elements is determined by subtracting the group number from the number 8.
Thus, sulfur (an element of group VIA) exhibits the lowest oxidation state -2, nitrogen (an element of group V) - the lowest oxidation state -3.
Based on the above rules, you can find the oxidation state of an element in any substance.
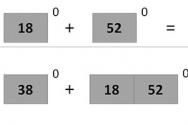
$+1 + x = 0 \hspace(1.5cm) +2 + 2x = 0 \hspace(1.5cm) +3 + 3x = 0$
$x = - 1 \hspace(2.3 cm) x = - 1 \hspace(2.6 cm) x = - 1$
$\overset(x)(Cl\overset(-2)(O_3))^(-1)$
The ability to find the oxidation state of chemical elements is a prerequisite for successfully solving chemical equations that describe redox reactions. Without it, you will not be able to create an exact formula for a substance resulting from a reaction between various chemical elements. As a result, solving chemical problems based on such equations will be either impossible or erroneous.
The concept of oxidation state of a chemical elementOxidation state is a conventional value with which it is customary to describe redox reactions. Numerically, it is equal to the number of electrons that an atom acquiring a positive charge gives up, or the number of electrons that an atom acquiring a negative charge attaches to itself.
In redox reactions, the concept of oxidation state is used to determine chemical formulas compounds of elements resulting from the interaction of several substances.
At first glance, it may seem that the oxidation number is equivalent to the concept of valence of a chemical element, but this is not so. Concept valence used to quantify electronic interactions in covalent compounds, that is, compounds formed by the formation of shared electron pairs. Oxidation number is used to describe reactions that lose or gain electrons.
Unlike valence, which is a neutral characteristic, the oxidation state can have a positive, negative, or zero value. A positive value corresponds to the number of electrons donated, and negative number attached. A value of zero means that the element is either in its elemental form, has been reduced to 0 after oxidation, or has been oxidized to zero after a previous reduction.
How to determine the oxidation state of a specific chemical element
Determining the oxidation state for a specific chemical element is subject to the following rules:
- The oxidation state of simple substances is always zero.
- Alkali metals, which are in the first group of the periodic table, have an oxidation state of +1.
- Alkaline earth metals, which occupy the second group in the periodic table, have an oxidation state of +2.
- Hydrogen in compounds with various non-metals always exhibits an oxidation state of +1, and in compounds with metals +1.
- The oxidation state of molecular oxygen in all compounds considered in school course inorganic chemistry is equal to -2. Fluorine -1.
- When determining the degree of oxidation in the products of chemical reactions, they proceed from the rule of electrical neutrality, according to which the sum of the oxidation states of the various elements that make up the substance must be equal to zero.
- Aluminum in all compounds exhibits an oxidation state of +3.
There are higher, lower and intermediate oxidation states. Highest degree oxidation, like valence, corresponds to the group number of a chemical element in the periodic table, but has a positive value. The lowest oxidation state is numerically equal to the difference between the number 8 group of the element. An intermediate oxidation state will be any number ranging from the lowest oxidation state to the highest.
To help you navigate the variety of oxidation states of chemical elements, we bring to your attention the following auxiliary table. Select the element you are interested in and you will receive the values of its possible oxidation states. Rarely occurring values will be indicated in parentheses.
To determine the conditional charge of atoms in redox reactions, use the table of oxidation of chemical elements. Depending on the properties of the atom, an element can exhibit a positive or negative oxidation state.
What is oxidation number
The conditional charge of the atoms of elements in complex substances is called the oxidation state. The charge value of atoms is recorded in redox reactions to understand which element is a reducing agent and which is an oxidizing agent.
The oxidation state is related to electronegativity, which shows the ability of atoms to accept or give up electrons. The higher the electronegativity value, the greater the ability of an atom to lose electrons in reactions.

Rice. 1. Electronegativity series.
The oxidation state can have three values:
- zero- the atom is at rest (all simple substances have an oxidation state of 0);
- positive- the atom gives up electrons and is a reducing agent (all metals, some non-metals);
- negative- the atom accepts electrons and is an oxidizing agent (most nonmetals).
For example, the oxidation states in the reaction of sodium with chlorine are as follows:
2Na 0 + Cl 2 0 → 2Na +1 Cl -1
In the reaction of metals with non-metals, the metal is always the reducing agent and the non-metal is the oxidizing agent.
How to determine
There is a table that shows all the possible oxidation states of elements.
|
Name |
Symbol |
Oxidation state |
|
Beryllium |
||
|
1, 0, +1, +2, +3 |
||
|
4, -3, -2, -1, 0, +2, +4 |
||
|
3, -2, -1, 0, +1, +2, +3, +4, +5 |
||
|
Oxygen |
2, -1, 0, +1, +2 |
|
|
Aluminum |
||
|
1, 0, +1, +3, +5, +7, rarely +2 and +4 |
||
|
Manganese |
2, +3, +4, +6, +7 |
|
|
2, +3, rarely +4 and +6 |
||
|
2, +3, rarely +4 |
||
|
2, rarely +1, +3, +4 |
||
|
1, +2, rarely +3 |
||
|
3, rarely +2 |
||
|
Germanium |
||
|
3, +3, +5, rarely +2 |
||
|
2, +4, +6, rarely +2 |
||
|
1, +1, +5, rarely +3, +4 |
||
|
Strontium |
||
|
Zirconium |
4, rarely +2, +3 |
|
|
3, +5, rarely +2, +4 |
||
|
Molybdenum |
3, +6, rarely +2, +3, +5 |
|
|
Technetium |
||
|
3, +4, +8, rarely +2, +6, +7 |
||
|
4, rarely +2, +3, +6 |
||
|
Palladium |
2, +4, rarely +6 |
|
|
1, rarely +2, +3 |
||
|
2, rarely +1 |
||
|
3, rarely +1, +2 |
||
|
3, +3, +5, rarely +4 |
||
|
2, +4, +6, rare |
||
|
1, +1, +5, +7, rarely +3, +4 |
||
|
Praseodymium |
||
|
Promethium |
||
|
3, rarely +2 |
||
|
3, rarely +2 |
||
|
Gadolinium |
||
|
Dysprosium |
||
|
3, rarely +2 |
||
|
Ytterbium |
3, rarely +2 |
|
|
5, rarely +3, +4 |
||
|
Tungsten |
6, rarely +2, +3, +4, +5 |
|
|
2, +4, +6, +7, rarely -1, +1, +3, +5 |
||
|
3, +4, +6, +8, rarely +2 |
||
|
3, +4, +6, rarely +1, +2 |
||
|
2, +4, +6, rarely +1, +3 |
||
|
1, +3, rarely +2 |
||
|
1, +3, rarely +2 |
||
|
3, rarely +3, +2, +4, +5 |
||
|
2, +4, rarely -2, +6 |
||
Or use this version of the table in your lessons.

Rice. 2. Table of oxidation states.
In addition, the oxidation states of chemical elements can be determined from the periodic table of Mendeleev:
- the highest degree (maximum positive) coincides with the group number;
- to determine the minimum value of the oxidation state, eight is subtracted from the group number.

Rice. 3. Periodic table.
Most nonmetals have positive and negative oxidation states. For example, silicon is in group IV, which means its maximum oxidation state is +4 and minimum -4. In compounds of non-metals (SO 3 , CO 2 , SiC), the oxidizing agent is a non-metal with a negative oxidation state or with a high electronegativity value. For example, in the compound PCl 3, phosphorus has an oxidation state of +3, chlorine -1. The electronegativity of phosphorus is 2.19, chlorine is 3.16.
The second rule does not work for alkali and alkaline earth metals, which always have one positive oxidation state equal to the group number. Exceptions are magnesium and beryllium (+1, +2). Also have a constant oxidation state:
- aluminum (+3);
- zinc (+2);
- cadmium (+2).
Other metals have a variable oxidation state. In most reactions they act as a reducing agent. In rare cases, they can be oxidizing agents with a negative oxidation state.
Fluorine is the most powerful oxidizing agent. Its oxidation state is always -1.
What have we learned?
From the 8th grade lesson we learned about the degree of oxidation. This is a conventional value showing how many electrons an atom can give or take during a chemical reaction. The value is related to electronegativity. Oxidizing agents accept electrons and have a negative oxidation state, while reducing agents donate electrons and exhibit a positive oxidation state. Most metals are reducing agents with a constant or variable oxidation state. Nonmetals can exhibit oxidizing and reducing properties depending on the substance with which they react.
Test on the topic
Evaluation of the report
Average rating: 4.7. Total ratings received: 146.
The formal charge of an atom in compounds is an auxiliary quantity; it is usually used in descriptions of the properties of elements in chemistry. This conventional electric charge is the oxidation state. Its value changes as a result of many chemical processes. Although the charge is formal, it clearly characterizes the properties and behavior of atoms in redox reactions (ORR).
Oxidation and reduction
In the past, chemists used the term "oxidation" to describe the interaction of oxygen with other elements. The name of the reactions comes from the Latin name for oxygen - Oxygenium. Later it turned out that other elements also oxidize. In this case, they are reduced - they gain electrons. Each atom, when forming a molecule, changes the structure of its valence electron shell. In this case, a formal charge appears, the magnitude of which depends on the number of conventionally given or accepted electrons. To characterize this value, the English chemical term “oxidation number” was previously used, which translated means “oxidation number”. When using it, it is based on the assumption that the bonding electrons in molecules or ions belong to an atom with a higher electronegativity (EO) value. The ability to retain their electrons and attract them from other atoms is well expressed in strong nonmetals (halogens, oxygen). Strong metals (sodium, potassium, lithium, calcium, other alkali and alkaline earth elements) have the opposite properties.
Determination of oxidation state
The oxidation state is the charge that an atom would acquire if the electrons participating in the formation of the bond were completely shifted to a more electronegative element. There are substances that do not have a molecular structure (alkali metal halides and other compounds). In these cases, the oxidation state coincides with the charge of the ion. The conventional or real charge shows what process occurred before the atoms acquired their current state. The positive oxidation number is the total number of electrons that have been removed from the atoms. A negative oxidation number is equal to the number of electrons gained. By changing the oxidation state of a chemical element, one judges what happens to its atoms during the reaction (and vice versa). The color of a substance determines what changes have occurred in the oxidation state. Compounds of chromium, iron and a number of other elements, in which they exhibit different valencies, are colored differently.
Negative, zero and positive oxidation state values

Simple substances are formed by chemical elements with the same EO value. In this case, the bonding electrons belong to all structural particles equally. Consequently, in simple substances the elements are not characterized by an oxidation state (H 0 2, O 0 2, C 0). When atoms accept electrons or the general cloud shifts in their direction, charges are usually written with a minus sign. For example, F -1, O -2, C -4. By donating electrons, atoms acquire a real or formal positive charge. In the OF2 oxide, the oxygen atom gives up one electron each to two fluorine atoms and is in the O +2 oxidation state. In a molecule or polyatomic ion, the more electronegative atoms are said to receive all the bonding electrons.
Sulfur is an element exhibiting different valence and oxidation states
Chemical elements of the main subgroups often exhibit a lower valency equal to VIII. For example, the valence of sulfur in hydrogen sulfide and metal sulfides is II. An element is characterized by intermediate and highest valence in the excited state, when the atom gives up one, two, four or all six electrons and exhibits valences I, II, IV, VI, respectively. The same values, only with a minus or plus sign, have the oxidation states of sulfur:
- in fluorine sulfide donates one electron: -1;
- in hydrogen sulfide the lowest value: -2;
- in dioxide intermediate state: +4;
- in trioxide, sulfuric acid and sulfates: +6.
In its highest oxidation state, sulfur only accepts electrons; in its lower state, it exhibits strong reducing properties. S+4 atoms can act as reducing agents or oxidizing agents in compounds, depending on the conditions.

Transfer of electrons in chemical reactions
When a crystal forms table salt sodium donates electrons to the more electronegative chlorine. The oxidation states of elements coincide with the charges of the ions: Na +1 Cl -1. For molecules created by sharing and shifting electron pairs to a more electronegative atom, only the concept of formal charge is applicable. But we can assume that all compounds consist of ions. Then the atoms, by attracting electrons, acquire a conditional negative charge, and by giving them away, a positive charge. In reactions they indicate how many electrons are displaced. For example, in the carbon dioxide molecule C +4 O - 2 2, the index indicated in the upper right corner of the chemical symbol for carbon reflects the number of electrons removed from the atom. Oxygen in this substance is characterized by an oxidation state of -2. The corresponding index for the chemical sign O is the number of added electrons in the atom.

How to calculate oxidation states
Counting the number of electrons donated and gained by atoms can be time consuming. The following rules make this task easier:
- In simple substances, the oxidation states are zero.
- The sum of the oxidation of all atoms or ions in a neutral substance is zero.
- In a complex ion, the sum of the oxidation states of all elements must correspond to the charge of the entire particle.
- A more electronegative atom acquires a negative oxidation state, which is written with a minus sign.
- Less electronegative elements receive positive oxidation states and are written with a plus sign.
- Oxygen generally exhibits an oxidation state of -2.
- For hydrogen, the characteristic value is: +1; in metal hydrides it is found: H-1.
- Fluorine is the most electronegative of all the elements, and its oxidation state is always -4.
- For most metals, the oxidation numbers and valences are the same.

Oxidation state and valency
Most compounds are formed as a result of redox processes. The transition or displacement of electrons from one element to another leads to a change in their oxidation state and valence. Often these values coincide. The phrase “electrochemical valence” can be used as a synonym for the term “oxidation state”. But there are exceptions, for example, in the ammonium ion, nitrogen is tetravalent. At the same time, the atom of this element is in the -3 oxidation state. In organic substances, carbon is always tetravalent, but the oxidation states of the C atom in methane CH 4, formic alcohol CH 3 OH and acid HCOOH have different values: -4, -2 and +2.
Redox reactions

Redox processes include many of the most important processes in industry, technology, living and inanimate nature: combustion, corrosion, fermentation, intracellular respiration, photosynthesis and other phenomena.
When compiling OVR equations, coefficients are selected using the electronic balance method, which operates with the following categories:
- oxidation states;
- the reducing agent gives up electrons and is oxidized;
- the oxidizing agent accepts electrons and is reduced;
- the number of electrons given up must be equal to the number of electrons added.
The acquisition of electrons by an atom leads to a decrease in its oxidation state (reduction). The loss of one or more electrons by an atom is accompanied by an increase in the oxidation number of the element as a result of reactions. For redox reactions occurring between ions of strong electrolytes in aqueous solutions, the method of half-reactions rather than electronic balance is often used.
When defining this concept, it is conventionally assumed that the bonding (valence) electrons move to more electronegative atoms (see Electronegativity), and therefore compounds consist of positively and negatively charged ions. The oxidation state can be zero, negative or positive values, which are usually placed above the element symbol at the top.
A zero oxidation state is assigned to atoms of elements in a free state, for example: Cu, H2, N2, P4, S6. Those atoms towards which the connecting electron cloud (electron pair) shifts have a negative oxidation state value. For fluorine in all its compounds it is equal to −1. Atoms that donate valence electrons to other atoms have a positive oxidation state. For example, for alkali and alkaline earth metals it is equal to +1 and +2, respectively. In simple ions like Cl−, S2−, K+, Cu2+, Al3+, it is equal to the charge of the ion. In most compounds, the oxidation state of hydrogen atoms is +1, but in metal hydrides (their compounds with hydrogen) - NaH, CaH 2 and others - it is −1. Oxygen is characterized by an oxidation state of −2, but, for example, in combination with fluorine OF2 it will be +2, and in peroxide compounds (BaO2, etc.) −1. In some cases, this value can be expressed as a fraction: for iron in iron oxide (II, III) Fe 3 O 4 it is equal to +8/3.
The algebraic sum of the oxidation states of atoms in a compound is zero, and in a complex ion it is the charge of the ion. Using this rule, we calculate, for example, the oxidation state of phosphorus in orthophosphoric acid H 3 PO 4. Denoting it by x and multiplying the oxidation state for hydrogen (+1) and oxygen (−2) by the number of their atoms in the compound, we obtain the equation: (+1) 3+x+(−2) 4=0, whence x=+5 . Similarly, we calculate the oxidation state of chromium in the Cr 2 O 7 2− ion: 2x+(−2) 7=−2; x=+6. In the compounds MnO, Mn 2 O 3, MnO 2, Mn 3 O 4, K 2 MnO 4, KMnO 4, the oxidation state of manganese will be +2, +3, +4, +8/3, +6, +7, respectively.
The highest oxidation state is its greatest positive value. For most elements, it is equal to the group number in the periodic table and is an important quantitative characteristic of the element in its compounds. The lowest value of the oxidation state of an element that occurs in its compounds is usually called the lowest oxidation state; all others are intermediate. So, for sulfur, the highest oxidation state is +6, the lowest is −2, and the intermediate is +4.
Changes in oxidation states of elements by group periodic table reflects the frequency of their changes chemical properties with increasing serial number.
The concept of the oxidation state of elements is used in the classification of substances, description of their properties, compilation of formulas of compounds and their international names. But it is especially widely used in the study of redox reactions. The concept of “oxidation state” is often used in inorganic chemistry instead of the concept of “valency” (see.